One of the biggest concerns with upper respiratory tract infections, like the common cold or a nasal infection, is the potential for more serious deep lung secondary infections, like pneumonia. If these secondary infections occur, they often develop rather quickly. This is highly concerning for vulnerable populations, like children and the elderly, who are more at-risk from pneumonia and other serious deep lung infections.
But how these secondary deep lung infections occur so rapidly after the emergence of first symptoms in the upper nasal tract has remained unsolved by scientists and medical experts alike. A new study from South Dakota State University’s Jerome J. Lohr College of Engineering may have the answer. The findings are published in the journal PLOS One.
Saikat Basu is an associate professor in SDState’s Department of Mechanical Engineering. He directs the Biomedical and Bioinspired Fluid Dynamics Lab there. As a doctoral and postdoctoral researcher, Basu trained in classical fluid mechanics and biomathematical modeling—some of the most complex and abstract levels of mathematics and mathematical biophysics possible.
He began applying his expertise and research prowess to an equally complex subject: infection transmission in the human airways during the COVID-19 pandemic. Notably, in 2021, he produced a single-authored computational fluids study that used simulations of inhaled respiratory transport and integrated the findings with virological inputs to explain the number of virions it would take to cause a COVID infection. The work later got verified through follow-up biological studies by other groups and represented a significant breakthrough.
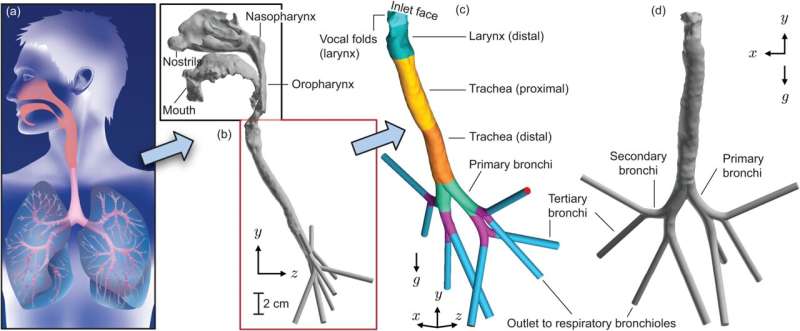
Since then, Basu has built on this research direction—based on syncing of fluid mechanics approaches with cross-cutting domains like virology, bacteriology and immunology—and has now produced a second single-authored study (a rarity in current academic research) that explains the mechanics behind secondary lung infections. This also marks Basu’s first work involving lung-level mechanics in the lower airway, with all his related prior studies being for the upper respiratory tract.
“Crafting single-author papers is a demanding endeavor, especially while managing the myriad responsibilities of faculty life. But the creative process can be supremely rewarding,” Basu said. “While interdisciplinary collaboration is essential as fields continue to converge, pursuing projects independently can deepen our thinking.”
How secondary lung infections develop
Larger microdroplets—typically greater than 10 micrometers (µm)—from the outside air rarely reach the bronchi as they will get trapped in filtration zones within the anterior nasal space before they can reach the lungs. These droplets often carry large viral loads that cause serious infections. They land along the upper airway, at sites such as the pharynx, triggering the initial infection and symptoms there.
Basu’s research has found that mucus fragments in the form of microdroplets from these initial infection sites can be inhaled rather easily downward into the lungs. The relatively straight structural shape of the laryngotracheal cavity helps the motion, enabling even the larger microdroplets to penetrate to the bronchial spaces.
These particles—some as large as 15 to 20 µm—form inside the upper airway by breaking off from the mucus substrate during inhalation. These droplets—with their origins at the initial infection sites—are virus-loaded and act as efficient carriers of pathogens to the lungs.
“This provides a plausible explanation for the brisk onset of secondary deep lung infections after initial upper respiratory tract infections,” Basu said.
The size of the particles that are reaching the lungs can potentially carry viral loads above the infectious threshold. As Basu notes, this can help explain the rapid progression of illnesses from the throat to the lungs.
Basu was able to analyze these mechanics by computationally simulating respiratory fluid physics within three-dimensional, computer-generated models of human airways built from CT scans. The results from this modeling were checked against reduced-order biomathematical estimates and existing experimental data.
This research helps explain why some respiratory infections can progress from mild illnesses to pneumonia within a very short time.
“This cannot be rationalized otherwise through, for example, tissue-level pathogen replication,” Basu said.
As Basu notes, these findings may help physicians predict the onset of secondary infections, which will help in protecting vulnerable populations.
Basu also expects this work to lay the foundation for a new avenue of bronchial biophysics research for his team.
More information:
Saikat Basu, On the mechanics of inhaled bronchial transmission of pathogenic microdroplets generated from the upper respiratory tract, with implications for downwind infection onset, PLOS One (2025). DOI: 10.1371/journal.pone.0335962
South Dakota State University
Citation:
Mechanics of mucus droplets explain swift spread of infections to lungs (2025, November 13)
retrieved 14 November 2025
from https://medicalxpress.com/news/2025-11-mechanics-mucus-droplets-swift-infections.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.